Insights on the Continuous-flow LVAD and Bicuspid Simulations
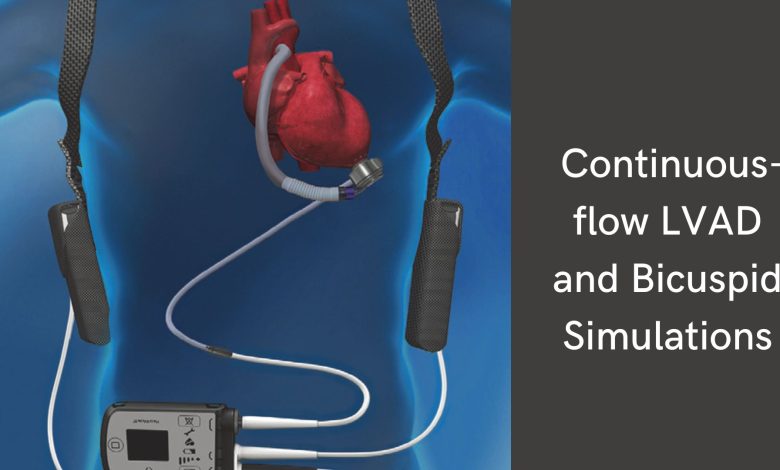
Introduction to LVAD simulator
A left ventricular assist device (LVAD) was initially developed in the 1960s. It is used to connect to cardiac transplants. The number of patients awaiting cardiac transplants has continued to rise. LVAD Simulation have also commonly become destination therapy. This device may also be used for temporary support. It can be used in cardiomyopathies. It is also useful for patients diagnosed with heart failure. As of 2019, there were an estimated 25,145 patients with LVAD devices. The number continues to grow. These patients are primarily managed at centers with LVAD-trained cardiologists and support staff.
Now an increasing number of patients rely on these devices. So it is inevitable that acute care will need to be provided outside of dedicated LVAD centers. Patients with LVAD are at high risk of experiencing a compilation. There are a variety of emergent complications associated with having an LVAD.
LVAD simulator is composed of the following 4 main components:
- The pump
– The pump sits internally and pumps blood from the left ventricle into the thoracic aorta - The driveline
– It is the percutaneous cable that exits the abdominal wall to connect the pump to the external components. - The external computer controller
– It monitors and controls pump performance with a display screen. - Power supply
– It typically rests along the patient’s hip with a backup battery located in an additional pouch.
The LVAD simulations clearly show how the LVAD pump provides continuous forward flow from the left ventricle to the aorta. Therefore there are notable physiological changes in these patients. There is no longer a definable systolic and diastolic component to the cardiac cycle.
So these patients will lack a palpable pulse. And healthcare providers will not be able to record traditional blood pressure. The low-cost simulator may be used to introduce these physiological changes. Also, provide training on common LVAD pathologies. Such as driveline site infections, bleeding events, and power source failure.
Discussion
The number of patients with LVADs will continue to grow. They will inevitably come under the care of healthcare providers unfamiliar with LVADs. Because patients with LVADs have frequent ED visits. and subsequent admissions with a wide array of emergent complications. These encounters represent HALO events for the majority of emergency physicians.
There are many aspects of managing LVAD patients that are unique and potentially unfamiliar to include techniques for measuring blood pressures and troubleshooting the device components. LVAD simulation training provides life-saving education and guidance on how to care for these patients.
With only minor modifications, LAVD simulation centers can create a functioning LVAD simulator that can be incorporated into their simulation curriculum.
Conclusions
The basic modifications outlined in this article allow for the creation of a high-fidelity low-cost LAVD physiology. Moreover, education on the complex pathology is unique to these patients. The model is both reusable and can be reverted back to the manikin’s original state with relative ease.
Introduction to bicuspid simulations
Bicuspid aortic valve anatomy has routinely been considered an exclusion in the setting of transcatheter aortic valve implantation. The large dimension of the aortic annulus has a more calcified, bulky, and irregular shape. A patient-specific computer simulation of transcatheter aortic valve replacement in the tricuspid aortic valve has been developed.
As a result, this can predict paravalvular regurgitation and conduction disturbance. We wished to validate a patient-specific computer simulation of TAVR in bicuspid aortic valves. Therefore, determining whether patient-specific transcatheter heart valve sizing and positioning might improve clinical outcomes.
Methods
Firstly, a retrospective study was performed on TAVR in bicuspid aortic valve patients. It had both pre and post-procedural computed tomography imaging. Preprocedural computed tomography imaging was used to create finite element models of the aortic root. Along with that the finite element analysis and computational fluid dynamics were performed. Moreover, the simulation output was compared with postprocedural computed tomography imaging, cine angiography, echocardiography, and electrocardiograms.
Therefore for each patient, multiple bicuspid simulations were performed. It helped to identify an optimal THV size and position for the patient’s specific anatomic characteristics.
Mathematical Modeling
As we discussed above the mathematical modeling of a simple and non-invasive method to comprehend hemodynamics. Along with the operating mechanism of the mechanical circulatory assist device. In one of the studies, a numerical model was developed to simulate hemodynamics under different conditions. Also, to evaluate the operating condition of a continuous-flow left ventricular assist device (LAVD). Therefore the numerical model consisted of a cardiovascular lumped parameter (CLP) model, a baroreflex model, and a LAVD model.
CLP Model
The CLP model was established to simulate the human cardiovascular system including the left heart, right heart system circulation, and pulmonary circulation. So the baroreflex model was used to regulate left and right ventricular end-systolic elastances, systemic vascular resistance, and heart rate.
Therefore the centrifugal pump HeartMate3 is used. It is used as an example to simulate the rotary pump dynamics at different operating speeds. LAVD simulation results show hemodynamics under normal left ventricular failure. Along with that, the different levels of pump support conditions can be reproduced by the numerical model.
Results of using HeartMate3
Based on simulation results, HeartMate3 operating speed can be maintained. Which is between 3600 rpm and 4400rpm to avoid pump regurgitation and ventricular suction.
Additionally, in the simulation system, the HeartMate3 speed should be between 3600rpm to 3800 rpm to provide optimal physiological perfusion. As a result, the developed numerical model is a feasible solution to simulate hemodynamics and evaluate the operating condition of continuous flow LAVD.
Conclusions
Patient-specific computer simulation of TAVR in bicuspid aortic valve through the bicuspid simulation may predict the development of important clinical outcomes. Which are paravalvular regurgitation and conduction abnormalities. So these patient-specific sizing and positioning may improve clinical outcomes of TAVR in bicuspid simulations of the bicuspid aortic valve.
Collective Results
A total of 37 patients were included in the study. The simulations accurately predicted the THV frame deformation. That is more than mild paravalvular regurgitation and major conduction abnormalities. And when compared with the implanted THV size and implant depth, optimal patient-specific THV sizing and positioning reduced simulation-predicted paravalvular regurgitation. Also reduced the markers of conduction disturbance.




